PreveCeutical Confirms Encouraging Results from Sol-gel Applicator Trials for Achieving Direct Nose-to-Brain Delivery in an Adult Human Nasal Cast
Vancouver, British Columbia: PreveCeutical Medical Inc. (the “Company” or “PreveCeutical”) (CSE: PREV, OTCQB: PRVCF, FSE: 18H), is pleased to provide an update on the soluble gel (“Sol-gel”) drug delivery research and development program (the “Sol-gel Program”), that involves testing and formulating an array of cannabis strains for the development and commercialisation of cannabinoid-based Sol-gels. The Sol-gels are administered using a custom applicator device (the “Sol-gel Applicator”) for direct and sustained nose-to-brain drug delivery.
Achieving patient benefit from use of medicinal cannabis for the management of central nervous system (the “CNS”) conditions, such as epilepsy, anxiety and depression, is reliant upon achieving effective and sustained delivery of the cannabis-based therapeutic to brain tissue. When taken orally medicinal cannabis can cause gastrointestinal upset, and when absorbed across the gut it is rapidly broken down by enzymes. As common drug delivery methods do not provide effective and sustained delivery to the brain, the delivery of cannabinoids to the CNS is a universal problem. PreveCeutical is pleased to announce that it expects to address this issue through its Sol-gel platform and Sol-gel Applicator device for direct nose-to-brain delivery of cannabis-based therapeutics.
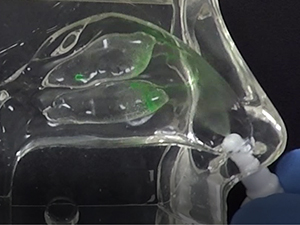
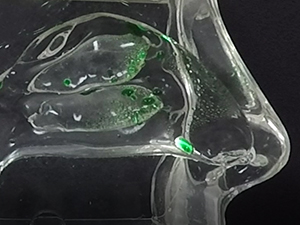
Traditional liquid nasal sprays are not retained in the nasal cavity for any appreciable time, and are only capable of generating random spray and deposition profiles as depicted in Figure 1a and Figure 1b, respectively.
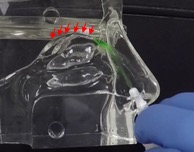
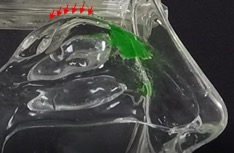
In contrast, the Company’s Sol-gel Applicator generates a precise spray profile that directs the Sol-gel formulation high into the upper nasal cavity (Figure 2a), where it remains following rapid gelation (Figure 2b). This is an important development, as the region targeted by the Sol-gel is adjacent to the olfactory epithelium (depicted by red arrows in the figures below), which serves as a direct passage for molecules into the brain.
To exemplify the Sol-gel and Sol-gel Applicator’s capabilities, an inhalation model was developed to mimic the inhalation action performed by patients when using nasal sprays, which combines an action of simultaneously spraying into a nostril accompanied by steady and deep nasal inhalation.
In the inhalation model, liquid sprays and Sol-gel sprays were compared for their delivery high into the nasal cavity along the target region, the olfactory epithelia. As shown in Figure 3a, the liquid spray profile shows random spray distribution with distinct large droplets, which is undesirable for the nose-to-brain delivery of therapeutics. In stark contrast, the Sol-gel formulation (Figure 3b) was drawn high into the nasal cavity, rapidly gelling and coating the target olfactory epithelium region. This encouraging development paves the way for the use of Sol-gels and the Sol-gel Applicator for direct nose-to-brain delivery.
The following video links showcase the contrasting spray profiles, in real-time, between a liquid and a Sol-gel being dispersed using the Sol-gel Applicator.
Video link to liquid spray profile demonstration
Video link to Sol-gel spray profile demonstration
The Company’s Chief Research Officer, Dr. Harendra Parekh stated, “This is an important development for PreveCeutical, having demonstrated that its engineered Sol-gels and applicator device achieve delivery and retention directly at target tissue high in the nasal cavity, a feat that cannot be claimed by liquid sprays existing in the market currently. What’s more exciting is that this outcome opens the door for PreveCeutical to potentially deliver a host of therapeutics from nose-to-brain in a reliable and sustained manner, which remains a significant challenge for many companies, globally.”
The CBD Sol-gel formulations, when used with the Company’s custom Sol-gel Applicator, will facilitate prolonged delivery of cannabinoids to patients’ central nervous system. The Company’s medicinal cannabis division expects to be able to offer a broad product range that can be tailored to specific medical conditions. For example, the Company is currently developing a CBD Sol-gel formulation with a cannabinoid composition designed to treat patient anxiety (see news release dated August 8, 2018).
About PreveCeutical
PreveCeutical is a health sciences company that develops innovative options for preventive and curative therapies utilising organic and nature identical products.
PreveCeutical aims to be a leader in preventive health sciences and currently has five research and development programs, including: dual gene therapy for curative and prevention therapies for diabetes and obesity; the Sol-gel Program; Nature Identical™ peptides for treatment of various ailments; non-addictive analgesic peptides as a replacement to the highly addictive analgesics such as morphine, fentanyl and oxycodone; and a therapeutic product for treating athletes who suffer from concussions (mild traumatic brain injury).
PreveCeutical sells CELLB9®, an Immune System Booster. CELLB9 is an oral solution containing polarized and potentiated essential minerals extracted from a novel peptide obtained from Caribbean Blue Scorpion venom. This product is available on the Company’s website.
For more information about PreveCeutical, please visit www.PreveCeutical.com, follow us on Twitter: http://twitter.com/PreveCeuticals and Facebook: www.facebook.com/PreveCeutical.
On Behalf of the Board of Directors
“Stephen Van Deventer”
Chairman, CEO and President
For further information, please contact:
Deanna Kress
Director of Corporate Communications & Investor Relations
+1-778-999-6063
deanna@PreveCeutical.com
Forward-Looking Statements:
This news release contains forward-looking statements and forward-looking information (collectively, “forward-looking statements”) within the meaning of applicable Canadian and U.S. securities legislation, including the United States Private Securities Litigation Reform Act of 1995. All statements in this news release that are not purely historical are forward-looking statements and include statements regarding beliefs, plans, expectations and orientations regarding the future including, without limitation, the completion of the Sol-gel Program, the development and testing of the Sol-gel formulations and the Sol-gel Applicator, the efficacy of the Company’s products, matters related to the Company’s current and planned research and development programs, including the Sol-gel Program, the Company’s anticipated business plans and its prospect of success in executing its proposed plans. Often, but not always, forward-looking statements can be identified by words such as “plans”, “expects”, “may”, “intends”, “anticipates”, “believes”, “proposes” or variations of such words including negative variations thereof and phrases that refer to certain actions, events or results that may, could, would, might or will occur or be taken or achieved. Actual results could differ from those projected in any forward-looking statements due to numerous factors including, risks and uncertainties relating to the inability of the Company, to, among other things, obtain any required governmental, regulatory or stock exchange approvals, permits, consents or authorizations required, including Canadian Securities Exchange acceptance of any planned future activities, commercialise therapeutic and diagnostic technologies, execute its proposed business plans, pursue business partnerships, complete its research programs as planned and obtain the financing required to carry out its planned future activities. Other factors such as general economic, market or business conditions or changes in laws, regulations and policies affecting the biotechnology, pharmaceutical or cannabis industries, may also adversely affect the future results or performance of the Company. These forward-looking statements are made as of the date of this news release and, unless required by applicable law, the Company assumes no obligation to update the forward-looking statements or to update the reasons why actual results could differ from those projected in these forward-looking statements. Although the Company believes that the statements, beliefs, plans, expectations, and intentions contained in this news release are reasonable, there can be no assurance that those statements, beliefs, plans, expectations, or intentions will prove to be accurate. Readers should consider all of the information set forth herein and should also refer to other periodic reports provided by the Company from time-to-time. These reports and the Company’s filings are available at www.sedar.com.
Readers are cautioned that forward-looking statements are not guarantees of future performance or events and, accordingly, are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty of such statements.